|

Highlights from the World Congress of Neurology 2021
Over 4400 delegates from 120 countries attended the XXV World Congress of Neurology 2021 held virtually on 3-7 October 2021 with the theme of “Inspired by the Past to build the Future of Neurology”. Our highlights include news of biomarker research to identify patients most likely to benefit from CGRP mAbs, discussions about ligand- and receptor-binding mAbs, and real-world data from different populations.
Presentations on the Congress website are available on demand for up to three months: https://wcn-neurology.com/.
|
Identifying patients most likely to respond to CGRP mAbs will be key to improving access to this important group of new drugs, and a study in Italy, Germany and Spain is investigating an array of demographic, clinical, psychological, cognitive, epigenetic, pharmacogenetic, biochemical and imaging markers. “We are not expecting to see a single biomarker that will predict response, there is more likely to be a panel of markers to help us separate likely responders from non-responders,” said Professor Cristina Tassorelli, from the University of Pavia, Italy.
|
|
 |
|
There is no difference in efficacy between ligand- and receptor-binding CGRP mAbs and there is no evidence that any one mAb works better than another, agreed speakers in response to questions at a joint session of the World Federation of Neurology and the International Headache Society. “Based on Phase 3 clinical studies, we don’t see any difference in efficacy between monoclonal antibodies that target the peptide or the receptor – they both end up inhibiting CGRP signalling,” said Professor Rami Burstein, from Harvard University, Boston, USA.
|
|

|
|
One in three patients treated with erenumab at an Italian centre reverted from chronic to episodic migraine after one month, and 71% by the end of one year of treatment, reported Dr Gloria Vaghi, from the University of Pavia, Italy.
|
|
 |
|
Reduction in migraine headache days in patients treated with fremanezumab in Korea compares well with reductions seen in the pivotal Phase 3 HALO studies in episodic and chronic migraine. Results of two phase 2b/3 studies in Korea, with the same design as the HALO trials, showed similar reductions in monthly headache days and responder rates to the international studies.
|
|
 |
|
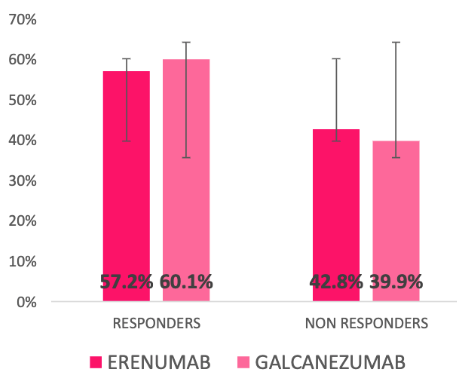
Nearly six out of 10 patients with chronic migraine had a significant clinical response to erenumab or galcanezumab in the first four weeks of treatment in a retrospective real world study reported from ASST Spedali Civili Brescia, Italy.
|
|
 |
|
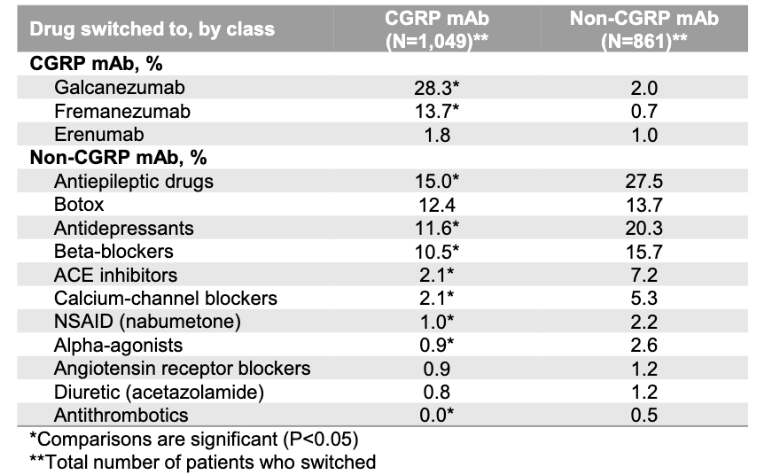
Patients taking CGRP mAbs are less likely to discontinue treatment within 12 months than those taking other approved preventive therapies for migraine, according to US claims data from over 4,500 patients prescribed CGRP mAbs and nearly 11,000 taking other preventives.
|
|
 |
For other recent news from the CGRP Forum, catch up at: News
If you’d like to contribute your experiences of using anti-CGRP therapies or share CGRP research findings, please get in touch.
|










