|

At this year’s virtual International Headache Congress, delegates accessed dozens of oral presentations and posters reporting latest insights on optimising use of anti-CGRP therapies. Our highlights focus on the growing body of real world evidence supporting treatment in high risk and hard-to-treat patients, as well as long term data, stopping treatment, and switching CGRP monoclonal antibodies. Full presentations on the Congress website are available on demand until 13 December 2021.
|
Real world studies are showing that CGRP mAbs are effective in significant patient groups excluded from randomised controlled trials (RCTs). They are also helping to identify predictors of treatment response and are extending understanding of safety issues.
|
|
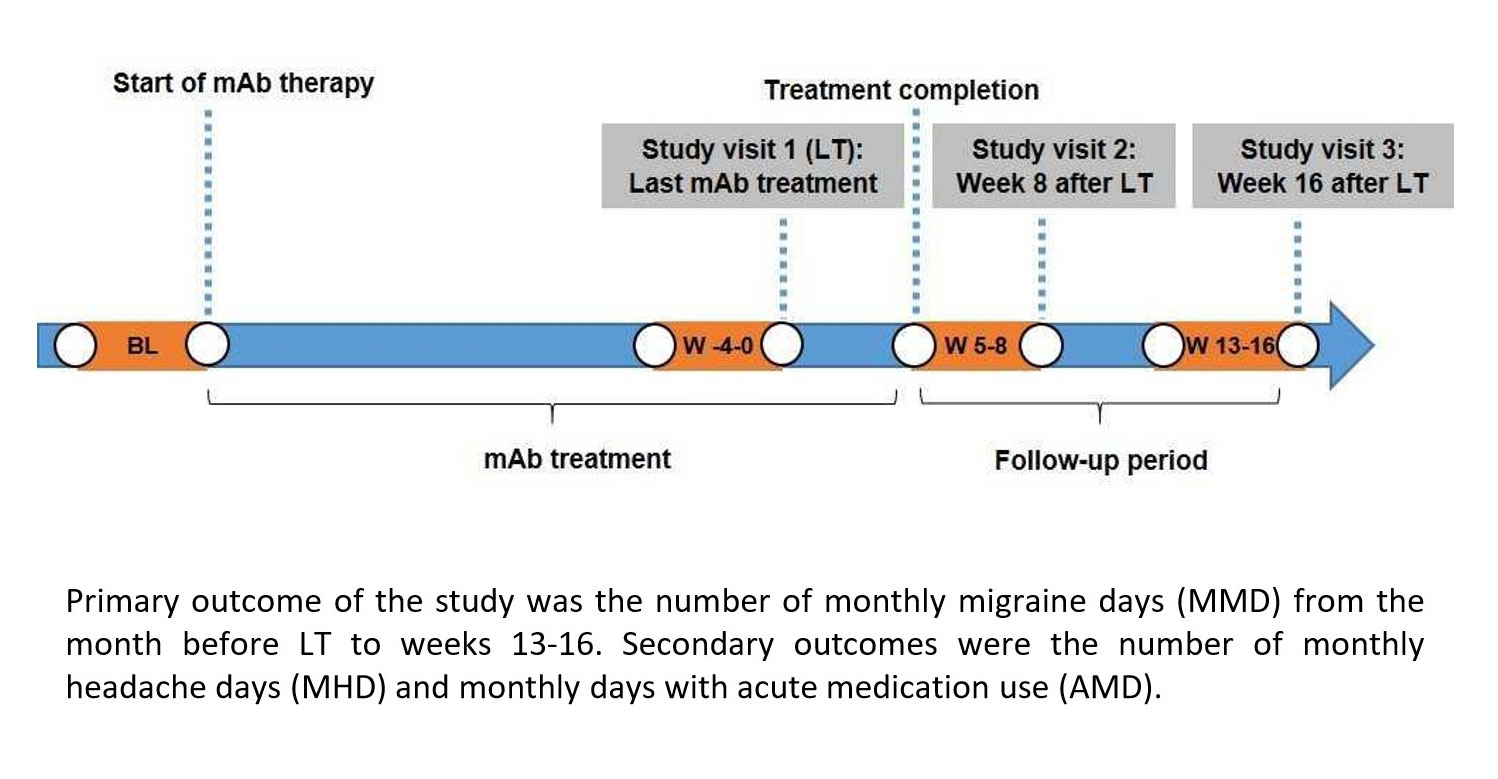
Stopping preventive treatment with CGRP mAbs typically leads to worsening migraine symptoms and, in some cases, a rebound effect, according to reports from clinicians in Germany, Portugal and Italy.
|
|
 |
|
Twelve month data from the HALO study and six month results from the FOCUS study have shown few blood pressure-related adverse events with fremanezumab and minimal changes in systolic blood pressure (SBP) or diastolic blood pressure (DBP).
|
|
Erenumab and galcanezumab have demonstrated similar efficacy and safety as preventive treatment in a very difficult-to-treat migraine population in Spain.
|
|
Long term acute treatment of migraine with rimegepant 75 mg as needed is associated with clinically relevant reductions in monthly migraine days (MMDs), according to post hoc results from an open label, 52-week safety study.
|
|
Real world experience from multiple new reports worldwide supports the use of CGRP mAbs in the treatment of cluster headache.
|
|
 |
|
No new safety signals have been identified in the ADVANCE open label extension study of atogepant 60 mg once daily for 40 weeks, with a 4-week safety follow-up period.
|
|
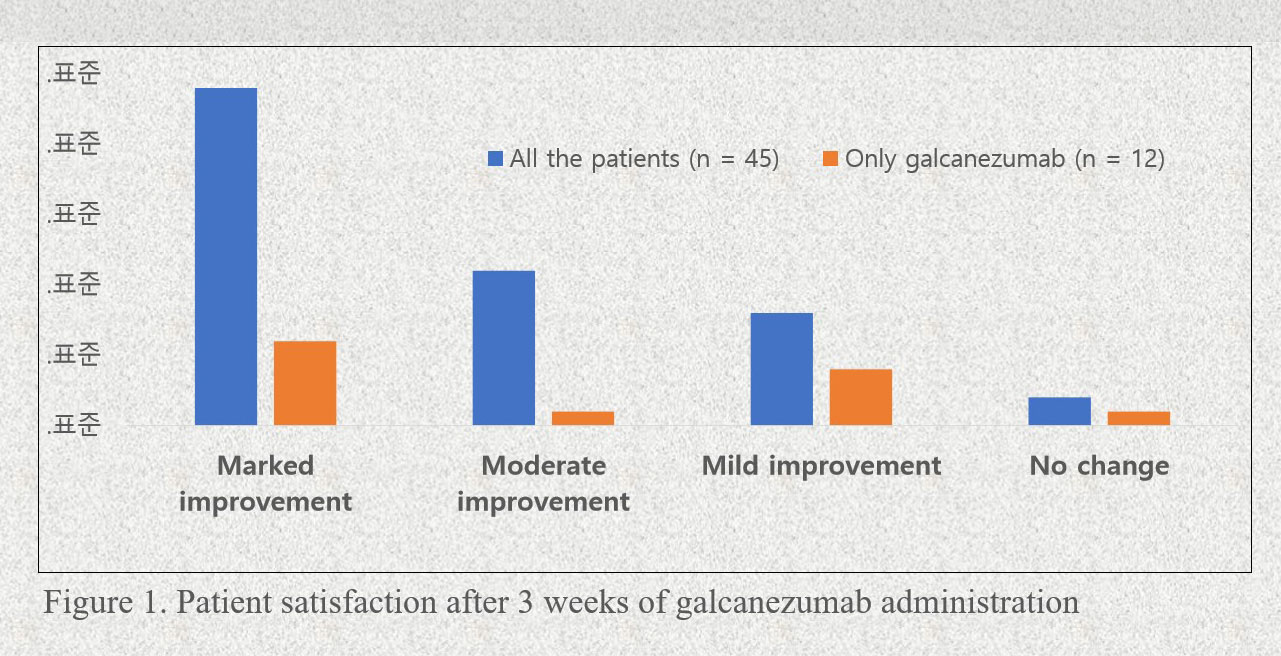
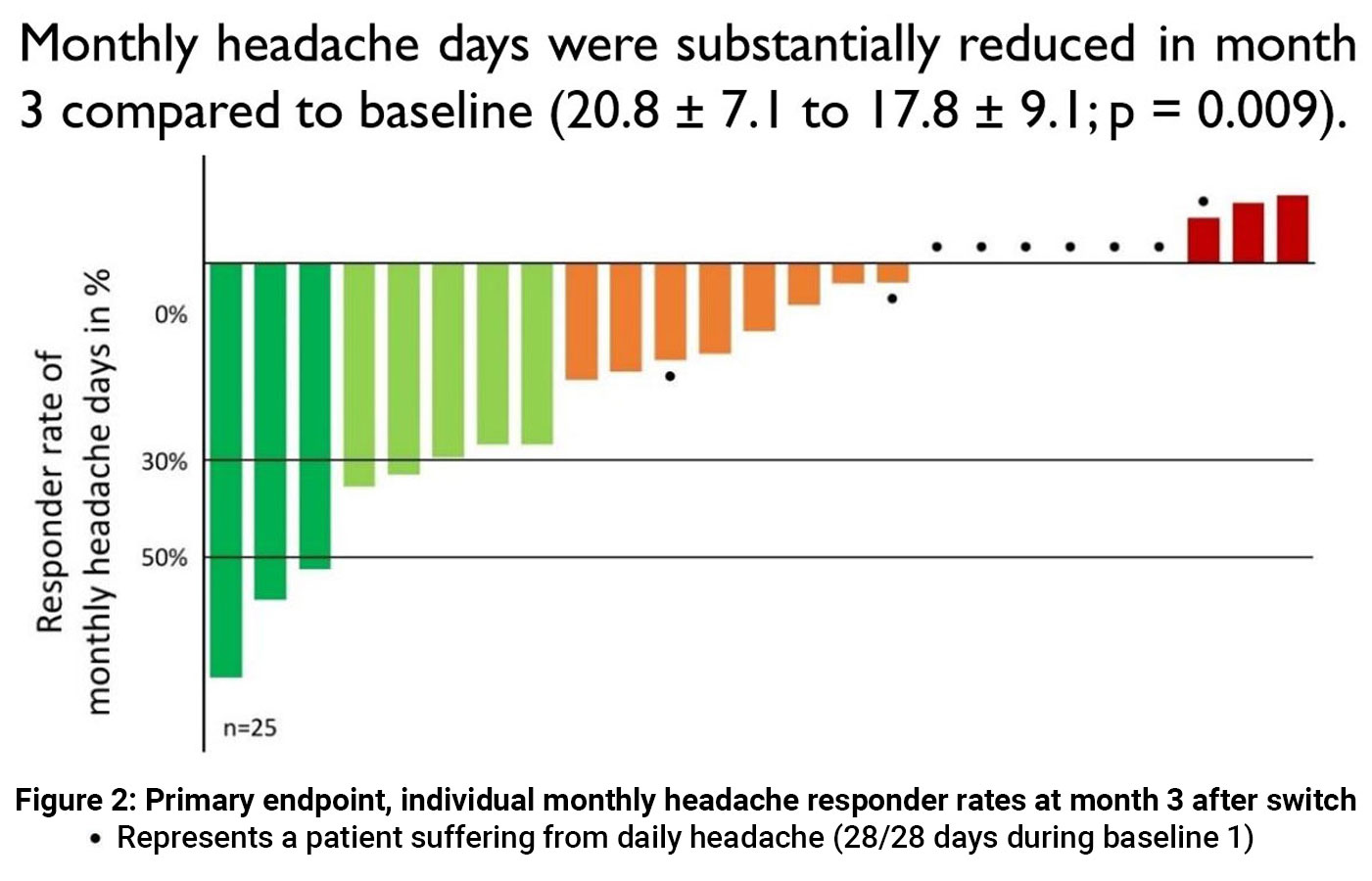
Approximately one in three non-responders to initial CGRP mAb treatment may benefit from switching to another antibody, according to results of two real world reports.
|
|
 |
|
High levels of patient satisfaction have been reported in an analysis of data from 302 US adult Migraine Buddy users who self-reported using at least four doses of ubrogepant, one in the preceding 14 days.
|
|
Long-term, real world experience with erenumab in high frequency episodic migraine (HFEM) and chronic migraine (CM) has shown sustained effectiveness, safety and tolerability in patients who failed on at least three previous preventive therapies.
|
For other recent news from the CGRP Forum, catch up at: News
If you’d like to contribute your experiences of using anti-CGRP therapies or share CGRP research findings, please get in touch.
|







